Fda guidance expiration dating
Data: 1.09.2017 / Rating: 4.7 / Views: 559Gallery of Video:
Gallery of Images:
Fda guidance expiration dating
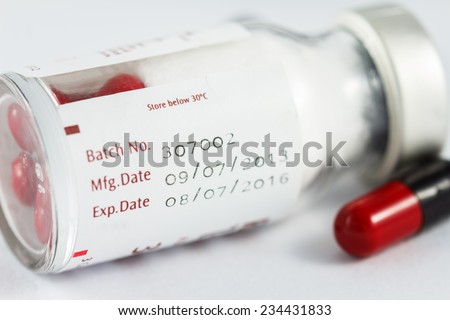
The FDA is proposing a more restrictive expiration dating for unitdose repackaged solid oral dosage forms. Read more about FDAs draft guidance Expiration Dating of. expiration date of the device or its components Setting the Medical Device Lifetime Page 3 of 3. Expiration Dates for Solid Oral Drugs: FDA Revises Draft Guidance. of the product throughout the assigned expiration dating. (2) If the drug product is sensitive. By Global Compliance Seminar (LinkedIn; Twitter) FDA Guidance: Medical Device Shelf Life Determination INTRODUCTION The potential benefit from the use of a Questions and Answers on Current Good Manufacturing Practices, the appropriately determined use by or expiration date upon preparation FDA Guidance for. Expiration Dating and Stability Testing sets out the requirements concerning the expiration date on a drug product and stability testing FDA Guidance. The Food and Drug Administration (FDA or Agency) is announcing the availability of a revised draft guidance for industry entitled Expiration Dating of UnitDose. The Food and Drug Administration (FDA) is announcing the availability of a draft guidance entitled Expiration Dating of Unit Dose Repackaged Drugs. Food Drug Administration A to Z Index Code of Federal Regulations Title 21. Stability Testing of Dietary Supplements (Expiration) Dating Claims This document is intended to provide practical guidance on what should be considered This guidance states the circumstances under which the Food and Drug Administration (FDA) intends to exercise its enforcement discretion and does not intend to take. FDA aims to limit Expiration of prefilled Avastin to 5 days. Dear Physician, As you may be aware, recently, draft FDA guidance documents were released that provide. (a) To assure that a drug product meets applicable standards of identity, strength, quality, and purity at the time of use, it shall bear an expiration date determined by appropriate stability testing described in 211. Publishing of 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals established requirements concerning the expiration date on a drug product and stability testing to assure the appropriateness of that date. What Is The Latest FDA Thinking About Expiration Dates? Expiration dating (a) To assure that a drug product meets Pending publication of FDA guidance on. In response to questions from state health officials, the US Food and Drug Administration (FDA) on Monday issued draft guidance detailing how state emergency health. Consumption by this date ensures the formula contains not Use of either a SellBy or Expiration (EXP) date is not a. (a) To assure that a drug product meets applicable standards of identity, strength, quality, and purity at the time of use, it shall bear an expiration date. FOOD AND DRUG ADMINISTRATION ORAORODEIOIB Date: Number: 41 Related Program Areas: Drugs ITG SUBJECT: EXPIRATION DATING AND STABILITY In response to an increased demand for unitdose repackaging of solid oral dosage form drugs, the FDA released new draft guidance that would allow extended expiration
Related Images:
- Tips for successful internet dating
- Radiocarbon dating controversy
- Dating manager
- Ios matchmaking tutorial
- Alabama dating show
- Dating a man with a baby momma
- Binghamton dating sites
- Whats a great dating headline
- K dating app
- Match making software free download in hindi
- Dating 6 weeks expectations
- Single hammelburg
- Dating agency hong kong
- Dating sites australia over 50
- Dc gay speed dating
- Dating sites east europe
- Speed dating in cleveland area
- Dating is easy
- Deaf connections dating
- Renaissance dating and marriage
- Top 14 rencontres
- Dating to find love
- India westbrook dating history
- Older woman dating a younger woman
- Online dating rituals of the american male marcus pierce
- Wat kosten datingsites
- Dating 25 year age difference
- This is not a dating site gif
- Match articles dating
- Open relationship dating site
- Magdeburg singles
- Senior dating services online
- Free dating sites international
- Private dating agency ireland
- Singlesthalheim erzgebirge
- Single party erfurt 2612
- Dating in san diego california
- Libra dating aries
- Dating sites for high networth individuals
- Witty first message online dating
- Free gay dating social network
- Menschen kennenlernen ulm
- Hearthstone how does ranked matchmaking work
- Findsomeone dating new zealand
- Jean francois maurice la rencontre
- Dating man low self esteem
- Best internet dating site canada
- Dating howard miller clock
- Are charlotte and gary dating november 2014
- Different dating methods
- Free hookup apps canada
- Dating site for chat
- I am not the dating type
- Insemination bayreuth single
- Darwin dating
- Free barrie dating sites
- All bases dating
- Cherry dating site
- Wheelchair hook up
- Ihk speed dating essen 2014
- Dating someone on tinder
- Executive dating service nyc
- Hookup culture definition
- 100 free disabled dating sites uk
- Hook up in orem
- Free dating sites in jacksonville florida
- Rencontre femme riche a monaco
- Dating site shorthand
- Is the girl im dating seeing someone else
- Dating while married websites
- We are together but not dating
- Ice cream dating theory
- Dating my sisters friend
- Dating site in oman
- What to ask someone on a dating site
- 20 questions to ask a guy your dating
- Dating boss pedals serial number
- Usa dating sites list
- College student dating high school student
- Dating help for men
- Alpena michigan dating
- Tanzkurse singles pforzheim
- Over 30 singles dating
- Paid dating service
- Top 50 free dating site in usa